
PANORAMIC Study – UK trial of COVID-19 antiviral treatments
 December 13th, 2021
December 13th, 2021 Nakita Cambow
Nakita Cambow Latest News
Latest News 0 Comments
0 Comments
The PANORAMIC study is a UK-wide clinical trial sponsored by the University of Oxford for COVID-19 antiviral treatments. The researchers are investigating treatments that can help people, in the community, with COVID-19 to get better early and reduce the need to be admitted to hospital.
 COVID-19 can cause great suffering, and it stops people from performing their daily activities, affecting their work, education, and caring responsibilities. The risk of complications from COVID-19 is increased in people with underlying health conditions, unvaccinated people, and those in whom the vaccine is less effective. In these people, COVID-19 can sometimes lead to significant medical problems, hospitalisation, and death.
COVID-19 can cause great suffering, and it stops people from performing their daily activities, affecting their work, education, and caring responsibilities. The risk of complications from COVID-19 is increased in people with underlying health conditions, unvaccinated people, and those in whom the vaccine is less effective. In these people, COVID-19 can sometimes lead to significant medical problems, hospitalisation, and death.
Vaccines remain the primary defence against COVID-19, but new antiviral treatment options for COVID-19 are being made available for groups at an increased risk of severe illness through the PANORAMIC study. These new treatments are used in the earliest stages of infection and often taken at home. These treatments should be administered as soon as possible after a confirmed COVID-19 positive PCR test has been received.
People who are eligible for the study are being strongly encouraged to sign up to help the UK gather data on how antivirals work in a predominantly vaccinated population. This will help the NHS to develop plans for making antivirals available to people who would benefit from them the most.
The purpose of this clinical trial is to find new treatments that help those suffering with COVID-19 at home and in the community get better quicker and without needing to be treated in hospital. Most people with COVID-19 are treated in the community and so we need to find treatments that are suitable for use in the community.
To be able to do this, the researchers aim to test individual possible treatments as soon as they become available. They are testing new antiviral treatments which might have beneficial effects for the treatment of COVID-19, but which may not yet have a license for use in the UK. All of the treatments in the PANORAMIC trial have been approved by the UK Medicines and Health Care Products Regulatory Agency (MHRA) for use in the study. The MHRA regulates the use of all medicines in the UK.
What is involved?
 PANORAMIC is a clinical trial open to those who meet the eligibility criteria. The trial will be mainly carried out remotely, this means you can participate from home, with all packs posted directly to you from the trial team. Questionnaires and diaries can be filled out online or, if needed, via phone call with one of the research team.
PANORAMIC is a clinical trial open to those who meet the eligibility criteria. The trial will be mainly carried out remotely, this means you can participate from home, with all packs posted directly to you from the trial team. Questionnaires and diaries can be filled out online or, if needed, via phone call with one of the research team.
The antiviral treatment currently being investigated is Molnupiravir. This antiviral oral capsule was approved by the UK’s medicines regulator (MHRA) on 4th November 2021.
Those who participate in the trial will either receive Usual Care, or Usual Care and an antiviral treatment. The study will use a computer programme to decide by chance whether you get the treatment or not. This process is completely automated based on your eligibility, the trial team cannot alter this.
If allocated to an antiviral treatment you will receive a trial pack from either the trial team or a local hub.
Those randomised to Usual Care, will receive an information booklet via email or post.
If you can access the internet, for the next 28 days you will be asked to complete an online diary of your symptoms and medical care you have received. If you can’t access the internet, the trial team can phone you every one to two weeks to get this information. The trial team will also contact you at three and six months after you started the trial, where they will ask you about any long-term COVID-19 symptoms.
You may withdraw from the trial at any stage by simply contacting the trial team.
How can I join the trial?
The trial is recruiting volunteers to join through the study website (HERE), participating GP practices, and other NHS Sites across the UK. Eligible patients will be contacted by the study team or a local healthcare professional (e.g. a GP or a research nurse) to consider enrolling in the study.
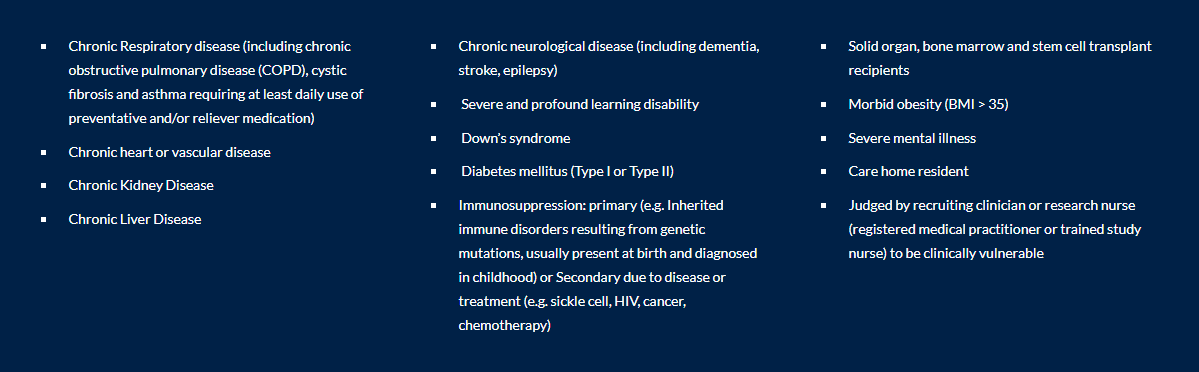
The PANORAMIC Trial is open to anyone meeting the following criteria:
- You are currently experiencing COVID-19 symptoms, beginning in the last 5 days.
- AND: You have had a positive PCR test for COVID-19;
- AND: You are aged 50 or over;
- OR: Aged 18 or over with a LISTED pre-existing condition
If you are interested in joining the study, you will need to answer some questions online or on the telephone to check you are suitable, and provide informed consent.
If you need to or would prefer you will be able to nominate a ‘study partner’ (family, friend, carer) to help you with the study.
Your GP, or a study nurse or doctor, will telephone you to make sure it is safe for you to be in the trial.
If you think you are eligible, would like to sign up and have not been invited to join, you can complete the online screening form by using the button below:


 ©2024 LUPUS UK (Registered charity no. 1200671)
©2024 LUPUS UK (Registered charity no. 1200671)