The Immune System and Lupus
This site is intended for healthcare professionals as a useful source of information on the diagnosis, treatment and support of patients with lupus and related connective tissue diseases.
Introduction
Systemic Lupus Erythematosus (SLE or lupus) is a chronic autoimmune rheumatic disease in which immunological abnormalities combine to cause inflammation in multiple organs and systems. This inflammation occurs as a result of the production of large amounts of autoantibodies (antibodies which attack the body’s own tissues) and the deposition of immune complexes (antibodies bound to antigen) into tissues. Hence, the mechanisms by which the immune system fails to distinguish between the body’s own tissues (self) and foreign organisms (e.g. bacteria, viruses - nonself), leading to the production of a wide spectrum of pathogenic autoantibodies and tissue pathology, are of particular interest. This chapter will consider what the normal immune system consists of (part 1) and then highlight our current understanding of abnormalities in the immune system which may contribute to the development of lupus (part 2).1. The Normal Immune System
The immune system provides a highly organised and versatile defence network essential to the health of an individual.There are three major components of the normal immune system.1. Cells which co-operate to eliminate invading pathogens and maintain internal order.
2. Substances produced by the immune system.
3.Organs closely linked with immune system (through which the immune cells pass).
The Cells of the Immune System
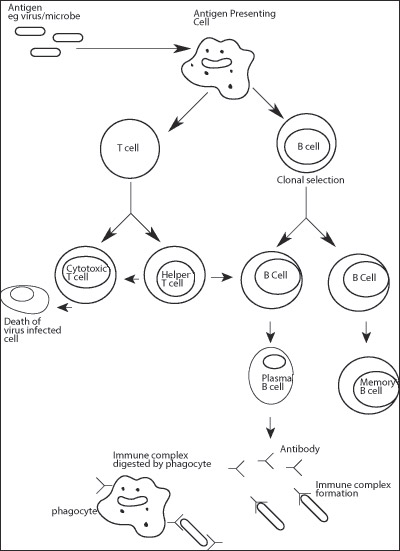
The immune system consists principally of the white blood cells (leukocytes), these have important roles in host defence and the generation of an immune response. White blood cells may be further divided into two types – myeloid cells (including macrophages and neutrophils) and lymphocytes (B cells, T cells and natural killer cells). Blood cells are produced to maturity in the bone marrow, before being released into the blood circulation. The exception to this is the T cell, which is produced in bone marrow but then migrates to the thymus gland (found in the neck) for maturation.
Neutrophils
Neutrophils constitute as much as two-thirds of all circulating white blood cells. Neutrophils are responsive to the presence of acute tissue inflammation. At such sites, the accumulation of these cells effects the process of phagocytosis (the surrounding and digesting of dead and dying tissues, bacteria, foreign particles etc.) and elimination of inflammatory agents.
Macrophages
These cells perform a variety of functions within the immune system.They are particularly effective in the presentation of antigens (various types of material including micro-organisms, foreign proteins and others) to B and T cells (described below) in order to facilitate antibody production targeted to the antigen. Macrophages, like neutrophils, are also important phagocytes.
B Cells
The defining feature of B cells is their unique ability to synthesise immunoglobulin. "Virgin" B cells are produced which are non-dividing. Once mature, these cells disperse into the circulation but have only a short life span (a few days) and most will undergo programmed cell death (also known as apoptosis). The presence of a foreign antigen initiates a process of activation in which a "virgin" B lymphocyte will undergo cell division and differentiation producing "memory" B cells and plasma cells. Immunoglobulin production in plasma cells leads to large quantities being secreted into the blood circulation with high specificity for the antigen.
T Cells
These cells are principally involved in facilitating or suppressing antibody production by B cells. There are two major types of T cells: helper and cytotoxic/suppressor, which can be distinguished by the presence of either a CD4 or CD8 cell-surface molecule. Helper T cells (CD4+) are involved in promoting antibody production by the B cells. Cytotoxic T cells have the ability to kill unwanted cells (such as viral infected cells). Suppressor T cells act to suppress antibody production in plasma cells and are, therefore, important in limiting the production of autoantibodies. Both cytotoxic and suppressor T cells bear the CD8 cell surface molecule. Natural killer [NK] cells make up a small proportion of white blood cells but play an important role in getting rid of virally infected cells and cancer cells.
Antigen Presenting Cells
The capture, processing and presentation of antigen to helper T cells is carried out by antigen presenting cells (APCs).These include macrophages and dendritic cells (which are shaped like an amoeba or an octopus!), found in the spleen and lymph nodes and B cells. This process is important since helper T cells are not able to recognise antigen independently. Instead, antigen must be associated with other molecules (known as MHC Class II) expressed on the surface of the antigen presenting cell.Antigen presenting cells capture and internalise antigen by a variety of methods including phagocytosis (see above). Once inside the cell, protein antigens are processed (cleaved into smaller peptides) and then associate with MHC Class II molecules. These are then transported to the cell surface where they are accessible for interaction with helper T cells.
The immune system consists principally of the white blood cells (leukocytes), these have important roles in host defence and the generation of an immune response. White blood cells may be further divided into two types – myeloid cells (including macrophages and neutrophils) and lymphocytes (B cells, T cells and natural killer cells). Blood cells are produced to maturity in the bone marrow, before being released into the blood circulation. The exception to this is the T cell, which is produced in bone marrow but then migrates to the thymus gland (found in the neck) for maturation.
Neutrophils
Neutrophils constitute as much as two-thirds of all circulating white blood cells. Neutrophils are responsive to the presence of acute tissue inflammation. At such sites, the accumulation of these cells effects the process of phagocytosis (the surrounding and digesting of dead and dying tissues, bacteria, foreign particles etc.) and elimination of inflammatory agents.
Macrophages
These cells perform a variety of functions within the immune system.They are particularly effective in the presentation of antigens (various types of material including micro-organisms, foreign proteins and others) to B and T cells (described below) in order to facilitate antibody production targeted to the antigen. Macrophages, like neutrophils, are also important phagocytes.
B Cells
The defining feature of B cells is their unique ability to synthesise immunoglobulin. "Virgin" B cells are produced which are non-dividing. Once mature, these cells disperse into the circulation but have only a short life span (a few days) and most will undergo programmed cell death (also known as apoptosis). The presence of a foreign antigen initiates a process of activation in which a "virgin" B lymphocyte will undergo cell division and differentiation producing "memory" B cells and plasma cells. Immunoglobulin production in plasma cells leads to large quantities being secreted into the blood circulation with high specificity for the antigen.
T Cells
These cells are principally involved in facilitating or suppressing antibody production by B cells. There are two major types of T cells: helper and cytotoxic/suppressor, which can be distinguished by the presence of either a CD4 or CD8 cell-surface molecule. Helper T cells (CD4+) are involved in promoting antibody production by the B cells. Cytotoxic T cells have the ability to kill unwanted cells (such as viral infected cells). Suppressor T cells act to suppress antibody production in plasma cells and are, therefore, important in limiting the production of autoantibodies. Both cytotoxic and suppressor T cells bear the CD8 cell surface molecule. Natural killer [NK] cells make up a small proportion of white blood cells but play an important role in getting rid of virally infected cells and cancer cells.
Antigen Presenting Cells
The capture, processing and presentation of antigen to helper T cells is carried out by antigen presenting cells (APCs).These include macrophages and dendritic cells (which are shaped like an amoeba or an octopus!), found in the spleen and lymph nodes and B cells. This process is important since helper T cells are not able to recognise antigen independently. Instead, antigen must be associated with other molecules (known as MHC Class II) expressed on the surface of the antigen presenting cell.Antigen presenting cells capture and internalise antigen by a variety of methods including phagocytosis (see above). Once inside the cell, protein antigens are processed (cleaved into smaller peptides) and then associate with MHC Class II molecules. These are then transported to the cell surface where they are accessible for interaction with helper T cells.
Substances Produced by the Immune System
AntibodiesThese molecules (also called immunoglobulin) are produced by plasma cells. Each molecule is composed of two distinct regions.The first, known as the Fc region, is similar in structure in all antibody molecules while the second, the Fab (a fragment which is antigen binding), provides structural specificity for a particular antigen. The production of an immune complex (antibody bound to an antigen) facilitates its destruction by immune cells such as phagocytes and cytotoxic T cells. Immune complexes involving self-antigens can lead to the destruction of the body's own tissues and, ultimately, to autoimmune disease.
Complement
The complement system consists of a family of proteins whose function is to facilitate the removal of micro-organisms and unwanted cells to which antibody has bound.These proteins are activated in a sequential order and some will bind to an antibody complexed with its target antigen. Activation of the complement system in turn leads to the activation of phagocytes. These cells contain cell surface molecules (receptors) which bind to complement on the surface of micro-organisms and unwanted cells, facilitating their removal. Abnormalities in the complement system (see later) feature in some lupus patients.
Cytokines
These are chemical messengers secreted by cells of the immune system (macrophages and lymphocytes) which act to coordinate the activities of immune cells during an immune response. Different cytokines have different biological effects on immune cells and are important in the process of immune regulation. Lupus is characterised by the production of high levels of various cytokines during active disease (discussed later), which may contribute to hyper-activation of the immune system.
2. The Immune System in Lupus
It is widely accepted that lupus arises as a result of a complex interaction of several factors: genetic, hormonal and environmental. The interaction of these factors in lupus leads to the production of pathogenic autoantibodies, deposition of immune complexes into tissues and, ultimately, widespread organ/system inflammation. This section considers how certain factors predispose an individual to developing autoimmunity and how abnormalities observed in the lupus immune system may be involved directly in the pathogenesis of lupus.Genetic Susceptibility
The great importance of an individual’s genetic makeup to any aspect of health cannot be over-emphasised and genetic makeup has an influence on the development of lupus. Recent investigations suggest that over 60 individual genes may contribute to the development of lupus. See chapter - Genetics
Sex Hormones
In healthy individuals, testosterone tends to suppress the immune system while oestrogen has an enhancing effect. The predominance of lupus in females over males [9:1] implies a role for sex hormones in this disease.
Studies in mice that develop a lupus-like disease have shown that sex hormones affect the disease. Thus, treatment of lupus-prone mice with testosterone can reduce lupus-like symptoms and giving additional oestrogen can make the disease worse. In human lupus, unique patterns of oestrogen production and metabolism have been reported involving increased oestrogen to androgen ratios and the preferential synthesis of the more potent immunomodulatory oestrogen types.An increase in levels of the hormone prolactin, which also has immunomodulatory effects, has correlated with active disease in lupus patients. In some female lupus patients, pregnancy exacerbates disease activity which may, conceivably, be due to increases in oestrogen and, subsequently, prolactin levels.
The Major Histocompatability Complex
Research into the involvement of genes which regulate the major histocompatibility complex (MHC) in human lupus has identified linked groups of genes (haplotypes) which are associated with disease susceptibility. For example, the haplotype HLA A1,B8,DR3 is present in 35% of caucasians with lupus. Antibodies to DNA, nuclear and cytoplasmic antigens in patients also correlate with MHC. It is, however, important to note that any correlations between MHC haplotype and lupus must be understood in relation to ethnic background.
Complement Deficiency
The presence of large amounts of circulating autoantibodies in lupus, which bind to self antigens, gives rise to the formation of immune complexes. Deposition of immune complexes on tissue surfaces can result in inflammation which, if perpetuated, may lead to tissue destruction. Complement deficiency, and thus deficiency in effective immune complex clearance by phagocytes, is a feature of lupus. Complement deficiency in lupus occurs for two reasons. If the disease is active, the complement factors are deposited in tissues like the kidney and the levels in the blood, therefore, go down. Secondly, hereditary and inborn abnormalities of the complement system causing an inability to make certain complement components (such as C1q, C1r, C1s or C4), or a reduction in complement receptors in phagocytes have also been identified in rare individuals with a lupus-like disease.
Cellular Abnormalities
Lupus is characterised by an overall shift towards cells supporting humoral (antibody-producing) responses and an impairment of cellular immunity. Enhanced B cell and T helper cell activity, reduced suppressor T cell activity and defects in cellular clearance of apoptotic material by phagocytes are the major cellular abnormalities observed in lupus.
B Cells
An increased number of antibody-producing B cells is observed in lupus patients compared to healthy controls and correlates directly with disease activity. Hypergammaglobulinemia is a characteristic feature of lupus and is, in part, a consequence of elevated levels of activated B cells. An important set of cells that regulate B cells [known as B regs] has been shown to be functionally deficient on lupus patients.
T Cells
Impaired T cell activation and activation induced cell death, together with increased apoptosis of natural killer T cells, have been reported in lupus. A reduction in numbers of circulating CD8 suppressor T cells are observed in patients with lupus and may explain the ineffective regulation of autoantibody producing B cells. In contrast an increase in numbers of circulating T cells providing help for antibody production (CD4+ T cells) is also found in patients with lupus.
Cytokines
Cytokines which promote help for antibody production (such as interleukin-6 and interleukin-10) are elevated in lupus, particularly in patients with active disease. Interleukin-2 production, a cytokine involved in regulating suppressor T cell activity (and thus autoantibody production), is notably decreased in lupus patients. More recently, an important role for enhanced interferon production in lupus has been suggested. A pathogenic role for cytokines in lupus is also supported by studies showing protective effects of neutralising antibodies to cytokines in both mouse models of lupus and in cultured blood cells from lupus patients. Of recent and particular interest, is a report of the development of systemic autoimmunity and lupus-like disease in mice lacking a gene (SOCS-1) critical in the regulation of several cytokines (including interferons, interleukin-6/10).
Impaired clearance of apoptotic material - a possible source of autoantigens
A link between complement deficiency and lupus led to the hypothesis that impairment in the clearance of apoptotic cells and immune complexes deposited into tissues may be important defects in lupus. In agreement with this hypothesis, studies have shown that many of the key autoantigens in lupus are present in surface blebs of apoptotic cells which have not been efficiently removed from circulation by phagocytes. This observation provides an immunopathological model in which inefficiently cleared apoptotic cells provide a source of key autoantigens in lupus which, in turn, leads to polyclonal B cell activation, autoantibody production and immune complex deposition. Inefficient clearance of immune complexes may result in inflammation which, if perpetuated, leads to tissue pathology. Furthermore, ultra-violet exposure increases the rate of cell apoptosis. This may well be the links between sun exposure and lupus. This model has led to the suggestion that lupus is “a disease of defective waste disposal”.
Autoantibodies
A wide spectrum of circulating antibodies which bind to self targets (particularly DNA) are found in patients with lupus. Autoantibodies to a variety of antigens (including nuclear, cytoplasmic and plasma membrane antigens) have been identified and some of these may be involved in tissue damage. Antibodies to double-stranded DNA are a hallmark of active lupus and appear to be involved in tissue destruction. Deposits of these antibodies alone or complexed with nucleosomes (DNA bound to histone proteins) have been identified in kidney biopsies from patients with glomerulonephritis, which has resulted from a previous local immune response leading to inflammation.
Summary
The clinical features of lupus are the consequence of its complex immunopathology, a combination of genetic, hormonal and environmental factors. The interaction of these factors leads to the production of pathogenic autoantibodies and the formation of immune complexes. Inappropriate control of cell mediated immune responses leads to ineffective clearance of autoantibody and immune complexes and possible widespread tissue and organ damage.
Dr Barry Ripley
Prof David Latchman
Medical Molecular Biology Unit
UCL Institute of Child Health, London
Prof David Latchman
Medical Molecular Biology Unit
UCL Institute of Child Health, London
Prof David Isenberg
Emeritus Professor of Rheumatology
The Centre of Rheumatology
University College London
Emeritus Professor of Rheumatology
The Centre of Rheumatology
University College London

 ©2024 LUPUS UK (Registered charity no. 1200671)
©2024 LUPUS UK (Registered charity no. 1200671)